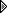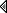Posts: 1,128
Threads: 45
Joined: Feb 2015
City: Roseville, MN
I'm in the process of cleaning a vernier dial assembly on my Philco 650 and decided to clean the brackets and metal dial hoop in the vinegar bath I was using to de-rust some metal pieces. The dial pieces were really gunked up. I was busy with company yesterday so I didn't get to looking at it till today. The vinegar had turned black. When I fished the parts out of the vinegar I discovered that the cross brace of the heavy wire hoop was gone. All that was left was the end pieces and part of the center hub. The remains were chalky and crumbling.

The one on the left is my spare, the right is the victim. There must have been a chemical reaction the vinegar had with the pot metal brace/hub that dissolved most of it. Just amazing. The hoop is nice and clean!
Posts: 713
Threads: 8
Joined: Apr 2018
City: S. Dartmouth
State, Province, Country: MA
Much of the base metal in "pot" metal is zinc, oops.

Pliny the younger
“nihil novum nihil varium nihil quod non semel spectasse sufficiat”
Posts: 16,495
Threads: 573
Joined: Oct 2011
City: Jackson
State, Province, Country: NJ
Yikes!
People who do not drink, do not smoke, do not eat red meat will one day feel really stupid lying there and dying from nothing.
Posts: 1,259
Threads: 32
Joined: Jan 2014
City: Wellborn Florida
Years ago was cleaning a fount from a very old Coleman lantern made of brass. The fount turned a nice copper color as the vinegar stripped the zinc out of the brass. Sprayed the lantern with clear coat looks nice, now a shelf Queen. Placed a note inside the burner cage saying danger do not light!
Posts: 1,848
Threads: 118
Joined: May 2008
City: Omak
State, Province, Country: WA
Hello David,
Yes ,you have to be careful about what we use to clean our anyiques.
Sincerely Richard
Posts: 4,875
Threads: 54
Joined: Sep 2008
City: Sandwick, BC, CA
Well vinegar is an acid, so anything soaking it it should be monitored, pot metal (probably Zamak metal) has zinc, and aluminum in it so both can corrode. Brass is usually all right with vinegar, even if it ends up coppery I've found that it's often just on the surface and will polish off. The point being if you are not sure what vinegar, or any other acid will do to a part, don't drop the part in a bucket of it and leave it for days unless it's a lump of heavy cast iron.




![[-] [-]](https://philcoradio.com/phorum/images/bootbb/collapse.png)